Our research group develops new desalination technology called desalination fuel cell. The desalination fuel cell is driven by chemical energy and provides both electricity and desalted water simultaneously, a functionality unique in the field of water desalination. In the future, this novel device could be a solution for potable water scarcity in off-grid countries and in households or small plants. This cell consists of an anode and a cathode separated by three channels and two ion exchange membranes. The anion and cation exchange membranes are separated by feedwater channel. On the opposite side of the anion exchange membrane is an anolyte channel, while a catholyte channel is on the opposite side of the cation exchange membrane. During discharge, reducing and oxidizing agents react spontaneously at the anode and cathode surfaces, respectively. This electrochemical reaction generates a spontaneous ionic current through the cell driving desalination in the feedwater channel.
Our research involves developing cutting-edge transport and thermodynamic mathematical models underpinning the performance of such cells. Our transport and thermodynamic models serve as a valuable tool to gain insight into the cell performance and to predict data and improve the performance of experimental cells. Thermodynamic analysis of chemical energy-driven desalination cells predicts fundamental outputs such as the maximum electricity production of such cells. Complementary transport modeling elucidates key factors affecting cell performance and factors limiting desalination performance and electricity generation. For example, figure 2 shows model predictions for sodium (fig 2.a) and chloride (fig 2.b) removal from the feedwater. Predictions demonstrate up to an order of magnitude reduction in NaCl concentration from inlet to outlet with a 500mM NaCl feed simultaneously to electricity generation with up to ~42 mW/cm2.
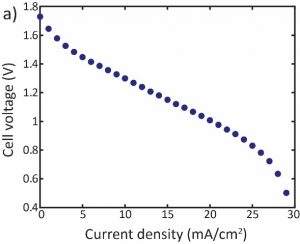
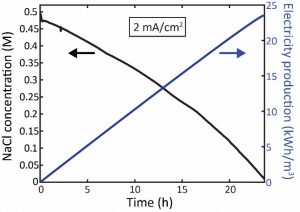
Experiments are performed using a prototype cell shown schematically in figure 1a, and pictured in figure 1b. Polarization curve experiments (fig. 2a) and constant current experiment (fig. 2b) are operated to examine and characterize the cell performance. Fig.3 shows the ability of such cell to desalinate feed water (0.5M NaCl solution, simulating seawater) to near zero concentration, and to produce electricity up to 23 kWh/m3 of desalted water.
Figure 3